| Figure IV: Rabies FA tests | |
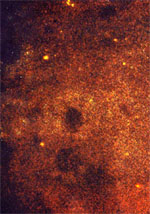 Negative result |
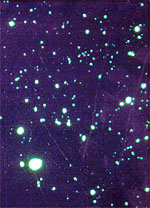 Positive result |
What animals will be tested? The local health department should be contacted whenever testing is considered to help determine if such testing is indicated, and if so, to arrange for shipment to the appropriate laboratory. In general, the following animals will be tested at the Washington Public Health Laboratories (PHL): 1) mammals that bite/expose humans (except fully-vaccinated dogs, cats, ferrets, and caged animals raised indoors); 2) ill animals with signs of rabies based on a veterinarian's assessment; 3) bats that are found in a setting where contact with people may have unknowingly occurred (see evaluating encounters for details). Mammals -- particularly bats -- that may have exposed an incompletely vaccinated dog, cat, or ferret can be tested for a fee at the Oregon State University Diagnostic Laboratory (541-737-3261). Mammals that expose a fully-vaccinated domestic animal need not be tested.
| Testing of Animals from King County:
Animals from King County are tested at the SKC DPH Laboratory. For
questions about whether a King County animal should be tested, or
to make arrangements for testing, contact the SKC DPH at (206)
296-4774. |
What tests are performed at the Public Health Laboratory (PHL)?: The test of choice for detecting rabies in animals is the fluorescent rabies antibody (FA) test. A slide containing fresh brain tissue is stained with an anti-rabies antibody mixture. When viewed under a microscope using appropriate light, the antibodies will fluoresce if rabies virus is present. Any animal excreting virus in its saliva should have detectable virus in its brain. Occasionally, supplemental tests are performed as well. There are currently no satisfactory tests for diagnosing rabies in live animals. All give an unacceptable number of false-negative results.
What types of specimens are required for FA testing?: Rabies FA testing requires fresh, unfixed brain tissue. No living animal will be accepted for rabies diagnostic studies (except under special circumstances). For testing of bats, the entire animal should be sent to the PHL. For other species, just the animal's head and upper neck should be submitted. Because the brain, spinal cord, salivary glands, and saliva may contain rabies virus, only veterinarians, animal control officers, or others who have been appropriately trained (and adequately vaccinated) should remove animal heads. This work should be done in a properly ventilated area using adequate protective gear.
| Euthanizing a bat
that needs to be tested: Captured bats should be euthanized
before being shipped to the PHL. Many health departments work with
local veterinarians to safely and humanely euthanize bats. Contact
the local health department to determine who does this in your
area. If other means are unavailable, bats can be euthanized safely
by placing the container holding the bat in a freezer
(10o F or colder) for at least 4 hours. Most household
freezers are set at 8-10 o F. Bats can be kept in a
household freezer overnight if necessary. |
How should specimens be shipped to the PHL?: Contact the local health department to make the arrangements for shipping any specimens to the PHL. Only specimens sent under the auspices of the local health department will be accepted for testing. For after-hour emergencies or on weekends or holidays, the Communicable Disease Epidemiology Section can be contacted at (206) 361-2914 or toll-free at 1-877-539-4344. All specimens submitted to the PHL must be accompanied by a "Rabies Specimen Submission Form" and specific packaging and shipping labels. Special reusable specimen containers are available from the local health department.
What are the possible outcomes of the testing?: Rabies FA testing at the PHL can result in the following outcomes:
Positive: A "positive" specimen is one in which rabies virus antigens have been detected. Any animal with a confirmed positive rabies FA test is considered capable of transmitting rabies. The FA test is highly specific for rabies (other diseases do not cause a positive test).In addition, non-specific or indeterminant fluoresence patterns are found rarely. In such instances, additional studies will be performed. The local health department will be notified if this occurs.
Negative: A "negative" specimen is one in which adequate brain tissue was examined yet no rabies antigens were detected. A negative FA test performed with an adequate specimen reliably rules out the possibility that the animal tested was capable of transmitting rabies.
Unsatisfactory: An "unsatisfactory" test is one in which the specimen submitted was inadequate for testing.
What is the turnaround time for test results?: Normally, specimens that are received at the PHL on weekdays before 10 am are processed the same day. Results are generally available by 5 pm. Occasionally, because of heavy demand for testing, samples will be prioritized (based on the type and date of exposure and on the species involved) and some results may not be available until the next day. The local health department will be notified when this is the case. In emergencies, testing can be done during evenings and on weekends and holidays.
How are the test results disseminated?: The results of
rabies testing are reported by telephone to the local health
department the afternoon the testing is completed. The local health
department will then contact the individuals involved (and their
physicians or veterinarians if necessary). When testing is done
after normal work hours, the results are reported by the
Communicable Disease Epidemiology Section. A written report of all
test results is also mailed to the local health department.
| Figure IV: Rabies FA tests | |
 Negative result |
 Positive result |